by Alan McCann | Jan 22, 2019 | Uncategorized
Fibralign announced today that it has been awarded a Small Business Innovation Research (SBIR) Sequential Phase II (Phase IIS) from the Department of Defense (DoD) Defense Health Program (DHP). This two-year grant provides $996,609 in funding to continue advancing...

by Alan McCann | Jan 12, 2019 | Uncategorized
Fibralign received Certification Notification on January 21, 2019 from DEKRA Certification B. V. that the Company’s management system meets the requirements of EN ISO 13486:2016. This internationally agreed standard sets out the requirements for a quality...

by Alan McCann | Feb 17, 2018 | Uncategorized
Fibralign received Certification Notification on February 18, 2018 from notifying body DEKRA Certification B. V. that it’s management system meets the requirements of EN ISO 13486:2016:2012 + AC:2012. The scope of the certification is for the design,...

by Alan McCann | Nov 27, 2017 | Uncategorized
AngelMD,an investment and networking platform connecting innovative medical startups, physicians, investors, and industry partners, today announced that it has completed a Syndicate funding round for Fibralign, Inc. Fibralign produces therapeutic medical devices that...

by Alan McCann | May 24, 2017 | Uncategorized
Fibralign Corporation, a developer of advanced therapeutic medical devices, announces the appointment of Dimitris Dionysiou, Ph.D., M.D. as its Chief Medical Officer. Dr. Dionysiou joins the Company during the clinical phase for its first product, the BioBridge®...

by Alan McCann | Jun 22, 2016 | Uncategorized
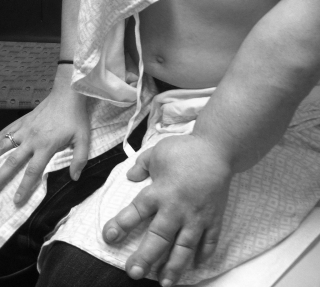
Researchers at the School of Medicine have developed a possible treatment for lymphedema, the severe swelling of an arm or leg that can occur when the lymph system is blocked. Using scaffolding composed of specially patterned collagen nanofibers, the researchers...