BioBridge
Engineered for Patient Care

The BioBridge® Collagen Matrix is an FDA cleared surgical mesh designed for use in soft tissue support and repair.
This thread-like device is made of medical-grade collagen, fabricated using Fibralign’s patented Nanoweave® technology. This novel product is the result of years of significant research collaboration with Stanford University and UCSF to optimize its nanostructure and parameters. BioBridge is designed to deliver the mechanical properties needed to provide support to weaknesses and deficiencies in soft tissue to support the body’s own repair process.
BioBridge is currently provided in single-use sterile “five-packs” (package containing five devices). BioBridge cannot be resterilized.

Proven Performance and Safety
BioBridge Up Close
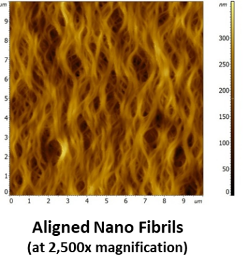

- Bioengineered native tissue structure produced containing only highly purified Type 1 porcine atelocollagen (pepsin treated)
- Bioengineered native tissue structure produced containing only highly purified Type 1 porcine atelocollagen (pepsin treated)
- Biocompatible, minimizes patient immunogenic response
- Design to integrate into tissue and safely fully resorb in 6 to 9 months
- Suturable – high biomechanical strength (30 MPa)
- Versatile – narrow ribbon form-factor provides flexibility in application Ready to use, provided in single-use sterile packaging (e-beam)
- Zero length crosslinking technology
- Pre-clinical data shows BioBridge helps to promote tissue repair, enhances natural maintenance process
BioBridge Up Close


Indications
The BioBridge Collagen Matrix is intended to reinforce soft tissue where weakness and deficiencies exist, specifically, for reinforcement of soft tissue repaired by sutures or suture anchors in tendon repair, including small tendons, ligaments; and general surgical procedures for tissue repair where weakness exists, including muscle flap reinforcement.
BioBridge is not intended to replace normal body structure or provide the full mechanical strength to support tendon repair.
Contraindications
BioBridge is contraindicated for use in any patient with known sensitivity to porcine products.
Use of this product in applications other than those indicated has the potential for serious complications, such as suture pullout or failure of the repair.
Proper surgical procedures and techniques are the responsibility of the medical professional. Each surgeon must evaluate the appropriateness of the procedure used based on the medical training and experience, along with the specific patient condition. Strict aseptic techniques should be followed.
Rx Only. For safe and proper use of this device, refer to Instructions For Use.
